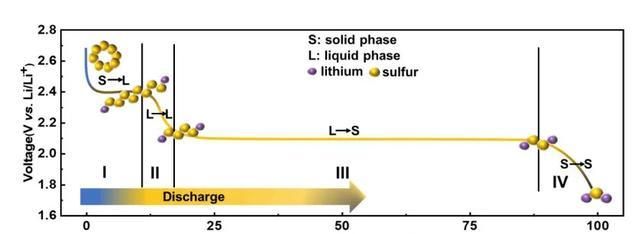
Lithium-sulfur batteries use sulfur as the electrode material, which is of great interest for its higher theoretical specific capacity and energy density compared to existing graphite-based lithium-ion batteries. During the discharge process, the S8 molecule gradually acquires electrons to combine with Li+ and undergoes multiple polysulfide intermediates (Li2Sx, x=2, 4, 6, 8) before it is finally converted to Li2S to complete the discharge process.
Equilibrium polysulfide adsorption seesaw: N and O double atoms suppress polysulfide shuttle effect diaphragm
Figure 1 Schematic diagram of sulfur species conversion during the discharge process
Unfortunately, as shown in Figure 1, the long-chain polysulfide intermediates (Li2Sx, x= 4, 6, 8) dissolve in the electrolyte, fall off from the cathode material, pass through the porous diaphragm, and reach the negative electrode of the battery, resulting in the loss of active material and collapse of the electrode structure. This process is called “polysulfide shuttle effect”, which is a key factor limiting the development of lithium-sulfur batteries. In order to solve this problem, many researchers have worked on the design of sulfur cathode, electrolyte modulation and diaphragm modification to suppress the “polysulfide shuttle effect”. However, the current literature reports that coated/intercalated modified diaphragms tend to block the diaphragm aperture, which to some extent limits the lithium ion transport and increases the internal resistance of the cell. Therefore, the development of novel diaphragm materials to modulate the polysulfide conversion behavior without affecting the lithium ion transport becomes a useful class of ideas for the modification of lithium-sulfur battery diaphragms.
Professor Zidong Wei and Associate Professor Cunpu Li of Chongqing University have reported lithium-sulfur battery diaphragms that can reversibly capture polysulfides using soft and hard acid-base theory without hindering lithium ion migration [1] (Small, 2018, 14(52): 1804277.). Recently, the team reported a lithium-sulfur battery diaphragm based on morpholine N and O diatomic chemisorption that can effectively suppress the “polysulfide shuttle effect” through further knowledge of the interfacial transition reaction [2] (ACS Appl. Mater. Interfaces, 2020, 10.1021 /acsami.0c04554). By analyzing the multi-step reduction process of sulfur monomer to Li2S, it can be found that the conversion of various types of soluble polysulfides involves several interfacial reactions, including solid-liquid interfacial reactions (S8-Li2S8), liquid-liquid interfacial reactions (Li2S8- Li2S4), and liquid-solid interfacial reactions (Li2S4- Li2S2). Since the battery has a constant plateau voltage with the continuous reduction of sulfur, the loss of all types of polysulfide intermediates will lead to the interruption of the subsequent sulfur reduction process, resulting in a decrease in battery capacity. Therefore, all types of polysulfides must be controlled equivalently to achieve the continuous and stable charging and discharging of lithium-sulfur batteries. Through the analysis of structural chemistry, it is known that the smaller the x of polysulfide Li2Sx, the closer the Li-S bond is to the ionic bond, i.e., the harder the acidity of lithium, and this change in the acidic hardness of Li itself makes it difficult to achieve equivalent capture of various polysulfides based on chemisorption of a single element.
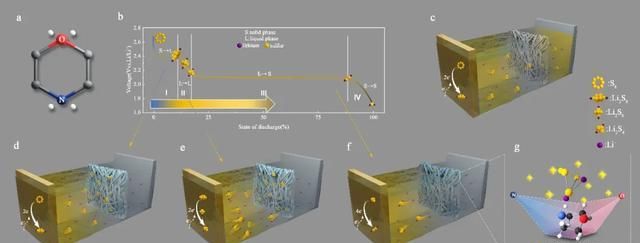
In this regard, as shown in Figure 2, the authors introduced morpholine as a capture unit on polypropylene diaphragms to inhibit polysulfide shuttling. The morpholine contains two heteroatoms, N and O. The more electronegative O atom has a concentrated charge and a high electron cloud hardness, while the less electronegative N atom has a dispersed charge and a low electron cloud hardness. The equivalent adsorption of polysulfides with different hardnesses was achieved under the modulating effect of the two atoms.
Equilibrium polysulfide adsorption seesaw: N and O double atoms suppress polysulfide shuttle effect septum
Fig. 2 (a) Chemical structure of morpholine; (b) variation process of polysulfide versus voltage during the discharge process; (c) PP septum cannot inhibit the diffusion of soluble polysulfide to the negative electrode; (d-f) morpholine-modified septum can effectively trap soluble polysulfide and subsequently release polysulfide on demand in the subsequent reduction process; (g) reversible polysulfide adsorption achieved by N,O double heteroatoms of morpholine ship conformation. equivalent adsorption.
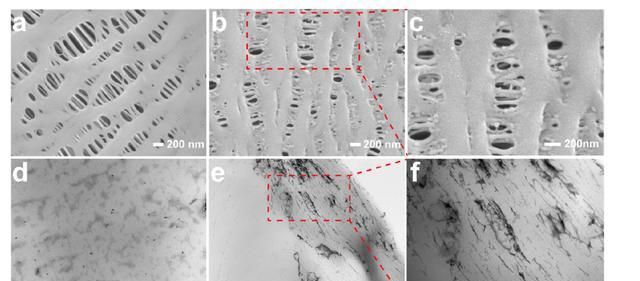
As shown in Figure 3, the synthetic strategy based on UV-assisted oxidation-polymerization coating-functionalization adopted by the authors can achieve the introduction of morpholine capture units while preserving the original porous structure of the septa. As the porous structure is successfully retained, the lithium ion transport will not be hindered, which is crucial for the multiplier charge/discharge performance of the battery.
Balancing the polysulfide adsorption seesaw: N and O diatoms suppress the polysulfide shuttle effect diaphragm
Figure 3 (a) SEM images of polypropylene porous diaphragm (PP); (b-c) SEM images of morpholine-modified diaphragm. After modification, the porous structure can be preserved; (d) TEM images of polypropylene porous diaphragm; (e-f) TEM images of morpholine-modified diaphragm with N and O heteroatoms stained by heavy metals, showing a darker color.
The authors used density flooding theory calculations to reveal the principle of equivalent homogeneous regulation of the polysulfide “shuttle effect” by this diaphragm structure. The binding energies of soluble polysulfides (Li2Sx, x = 4, 6, 8) to piperidine (only one N atom), tetrahydrofuran (only one O atom) and morpholine (N and O atoms) were investigated, as shown in Figure 4. Eb ≈ -1.5 eV). When the long-chain polysulfide passes through the morpholine side, the N and O heteroatoms in the morpholine will capture it to the middle of the morpholine ship conformation, forming a double-atom adsorption to prevent the loss of polysulfide, thus realizing the continuous and stable charging and discharging of lithium-sulfur batteries.
Balanced polysulfide adsorption seesaw: N and O double atoms suppress polysulfide shuttle effect diaphragm
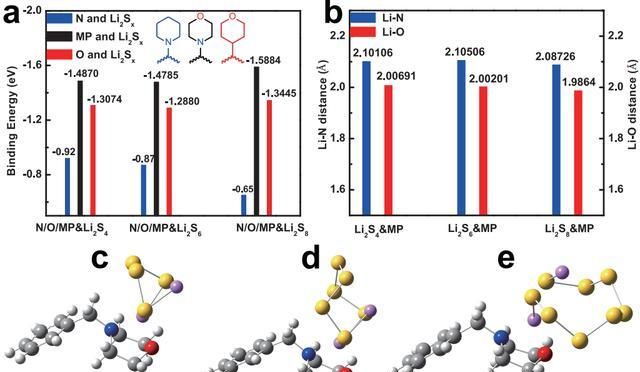
Fig. 4 (a) Binding energy between polysulfide and piperidine (N atom only), tetrahydrofuran (O atom only), and morpholine, where the diatomic adsorption is stronger than the single atomic adsorption of N, O. The diatomic adsorption of morpholine decreases the deviation of binding energy; (b) distance between Li-N and Li-O in the optimized system of morpholine-Li2Sx (x=4, 6, 8); (c-e) morpholine and Li2S4 (c), Li2S6(d), Li2S8(e) in the optimized structures to form boat conformations.
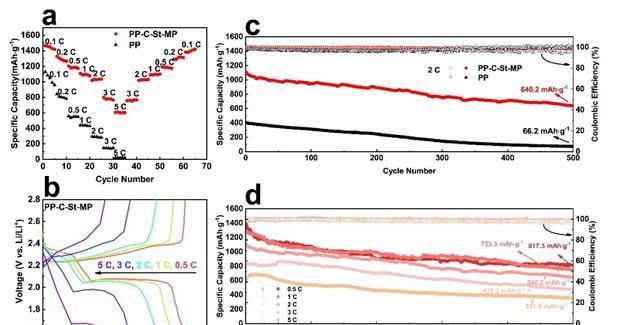
Thanks to the above reasons, the buckle cell with morpholine-modified diaphragm maintains a specific capacity of 640.2 mAh-g-1 for 500 cycles at 2 C, with good cycling stability and reversibility. The multiplicative performance of the modified diaphragm cell is also much better than that of the polypropylene porous diaphragm due to the protective effect on the electrode structure.
Balanced polysulfide adsorption seesaw: N and O diatoms suppress polysulfide shuttle effect diaphragm